1876 ⟶ Gibbs Publishes on the Equilibrium of Heterogeneous Substances
Josiah Willard Gibbs publishes On the Equilibrium of Heterog...Year
1871
1876
1877
1884
1923
⚛️ Boltzmann and Gibbs Develop Statistical Mechanics
89 – Ludwig Boltzmann & Josiah Willard Gibbs: Statistical mechanics (Boltzmann equation, 1872)⟶

Statistical MechanicsThermodynamicsBoltzmann EquationPhysics19th Century ScienceGibbsBoltzmannGas DynamicsEntropy
 Austria
Austria United States
United States⚖️ Gibbs Publishes on the Equilibrium of Heterogeneous Substances
Josiah Willard Gibbs publishes On the Equilibrium of Heterogeneous Substances, a compilation of his work on thermodynamics and physical chemistry which lays out the concept of free energy to explain the physical basis of chemical equilibria.⟶

ThermodynamicsPhysical ChemistryFree EnergyChemical EquilibriumGibbsPhase Rule19th Century Science
 United States
United States💨 Boltzmann Establishes Statistical Derivations
Ludwig Boltzmann establishes statistical derivations of many important physical and chemical concepts, including entropy, and distributions of molecular velocities in the gas phase.⟶

Statistical MechanicsEntropyBoltzmannThermodynamicsGas Dynamics19th Century SciencePhysical Chemistry
 Austria
Austria⚖️ Henry Louis Le Chatelier Develops Le Chatelier's Principle
Henry Louis Le Chatelier develops Le Chatelier's principle, which explains the response of dynamic chemical equilibria to external stresses.⟶
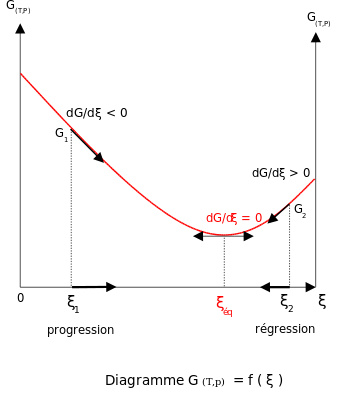
ChemistryPhysical ChemistryChemical EquilibriumThermodynamicsLe Chatelier's PrincipleHenry Louis Le Chatelier19th Century
 France
France⚛️ Lewis and Randall Publish Thermodynamics and the Free Energy of Chemical Substances
Gilbert N. Lewis and Merle Randall publish Thermodynamics and the Free Energy of Chemical Substances, first modern treatise on chemical thermodynamics.⟶

ThermodynamicsChemical ThermodynamicsFree EnergyPhysical ChemistryLewisRandall20th Century ScienceTreatise
 United States
United States