1924 ⟶ Pauli Formulates the Quantum Pauli Exclusion Principle
Wolfgang Pauli: quantum Pauli exclusion principleYear
1923
1924
1925
🔬 Stern–Gerlach Experiment Demonstrates Spatial Quantization

Quantum MechanicsAtomic PhysicsStern-Gerlach ExperimentSpatial QuantizationAtomic Structure1920sExperimental PhysicsMeasurement
 Germany
Germany👤 Pauli Formulates the Quantum Pauli Exclusion Principle
Wolfgang Pauli: quantum Pauli exclusion principle⟶
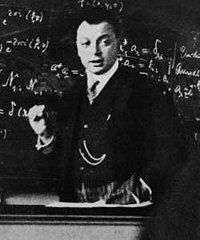
Quantum MechanicsPauli Exclusion PrincipleQuantum PhysicsAtomic PhysicsElectronsPauli1920sFundamental Particles
 Germany
Germany🌊 De Broglie Introduces the Wave-Model of Atomic Structure
Louis de Broglie introduces the wave-model of atomic structure, based on the ideas of wave–particle duality.⟶

Quantum MechanicsAtomic StructureWave-Particle DualityDe BroglieWave ModelQuantum Physics1920sTheoretical Physics
 France
France✨ Pauli Develops the Exclusion Principle
Wolfgang Pauli develops the exclusion principle, which states that no two electrons around a single nucleus may have the same quantum state, as described by four quantum numbers.⟶

Quantum MechanicsPauli Exclusion PrincipleQuantum PhysicsAtomic PhysicsElectronsPauli1920sAtomic Structure
 Germany
Germany📐 Schrödinger Publishes the Schrödinger Equation

Quantum MechanicsSchrödinger EquationQuantum PhysicsWave MechanicsSchrödinger1920sTheoretical PhysicsMathematical Physics
 Austria
Austria