1805 ⟶ Joseph Louis Gay-Lussac Determines Water Composition by Volume
Joseph Louis Gay-Lussac discovers that water is composed of ...Year
1803
1805
1808
1849
1884
💨 John Dalton Proposes Dalton's Law of Partial Pressures
John Dalton proposes Dalton's law, which describes relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.⟶

ChemistryDalton's LawGasesPressureDalton19th Century
 United Kingdom
United Kingdom💧 Joseph Louis Gay-Lussac Determines Water Composition by Volume
Joseph Louis Gay-Lussac discovers that water is composed of two parts hydrogen and one part oxygen by volume.⟶

ChemistryWaterGay-LussacCompositionVolume19th Century
 France
France🧪 John Dalton: Atomic Theory in Chemistry
John Dalton: Atomic Theory in (chemistry).⟶

ChemistryAtomic TheoryDaltonAtomsMatter19th Century
 United Kingdom
United Kingdom💨 Gay-Lussac's Gas Laws Investigations
Joseph Louis Gay-Lussac collects and discovers several chemical and physical properties of air and of other gases, including experimental proofs of Boyle's and Charles's laws, and of relationships between density and composition of gases.⟶

ChemistryPhysicsGas LawsBoyle's LawCharles's LawDensityJoseph Louis Gay-Lussac19th Century ScienceExperimental Physics
 France
France🧪 Louis Pasteur clarifies optical rotation and advances stereochemistry
Louis Pasteur discovers that the racemic form of tartaric acid is a mixture of the levorotatory and dextrotatory forms, thus clarifying the nature of optical rotation and advancing the field of stereochemistry.⟶

StereochemistryOptical IsomerismTartaric AcidLouis PasteurChemistryMolecular Structure19th Century
 France
France⚖️ Henry Louis Le Chatelier Develops Le Chatelier's Principle
Henry Louis Le Chatelier develops Le Chatelier's principle, which explains the response of dynamic chemical equilibria to external stresses.⟶
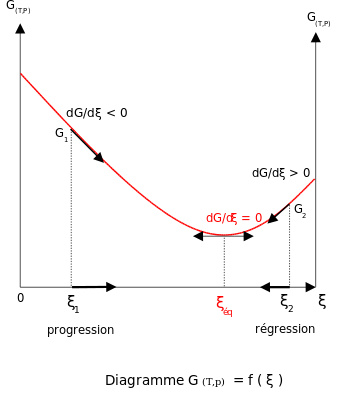
ChemistryPhysical ChemistryChemical EquilibriumThermodynamicsLe Chatelier's PrincipleHenry Louis Le Chatelier19th Century
 France
France